Magnesium Protons Neutrons Electrons Electron Configuration
Magnesium is an essential part of the human body and it is required for biochemical reactions in the body. Magnesium is the 11th abundant element (by mass) found in the human body. Out of the total amount of magnesium present in the human body, around 60% of magnesium is in the skeleton, 39% is in muscle tissues and 1% being extracellular.
Mg Periodic Table Protons Neutrons And Electrons Awesome Home
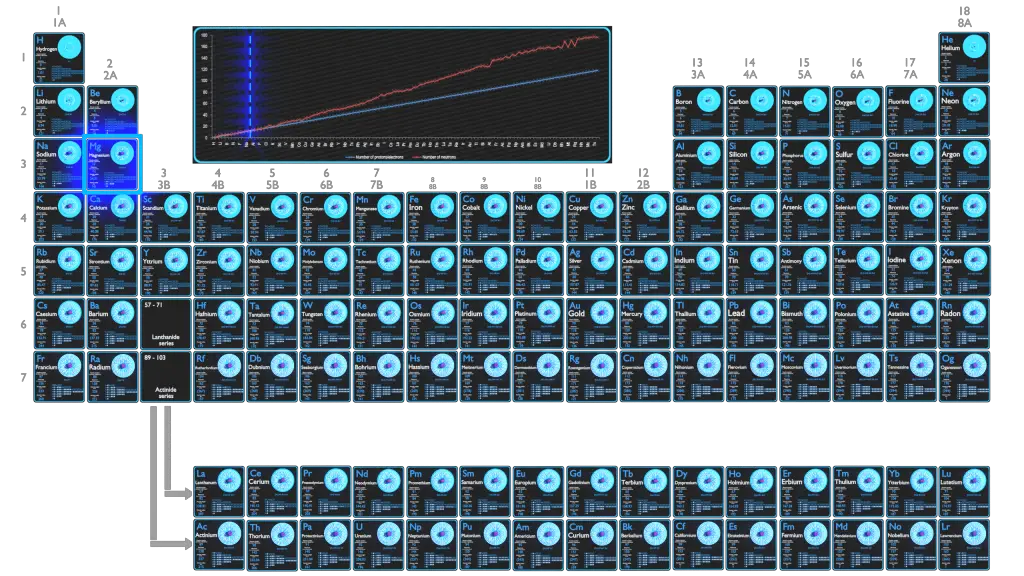
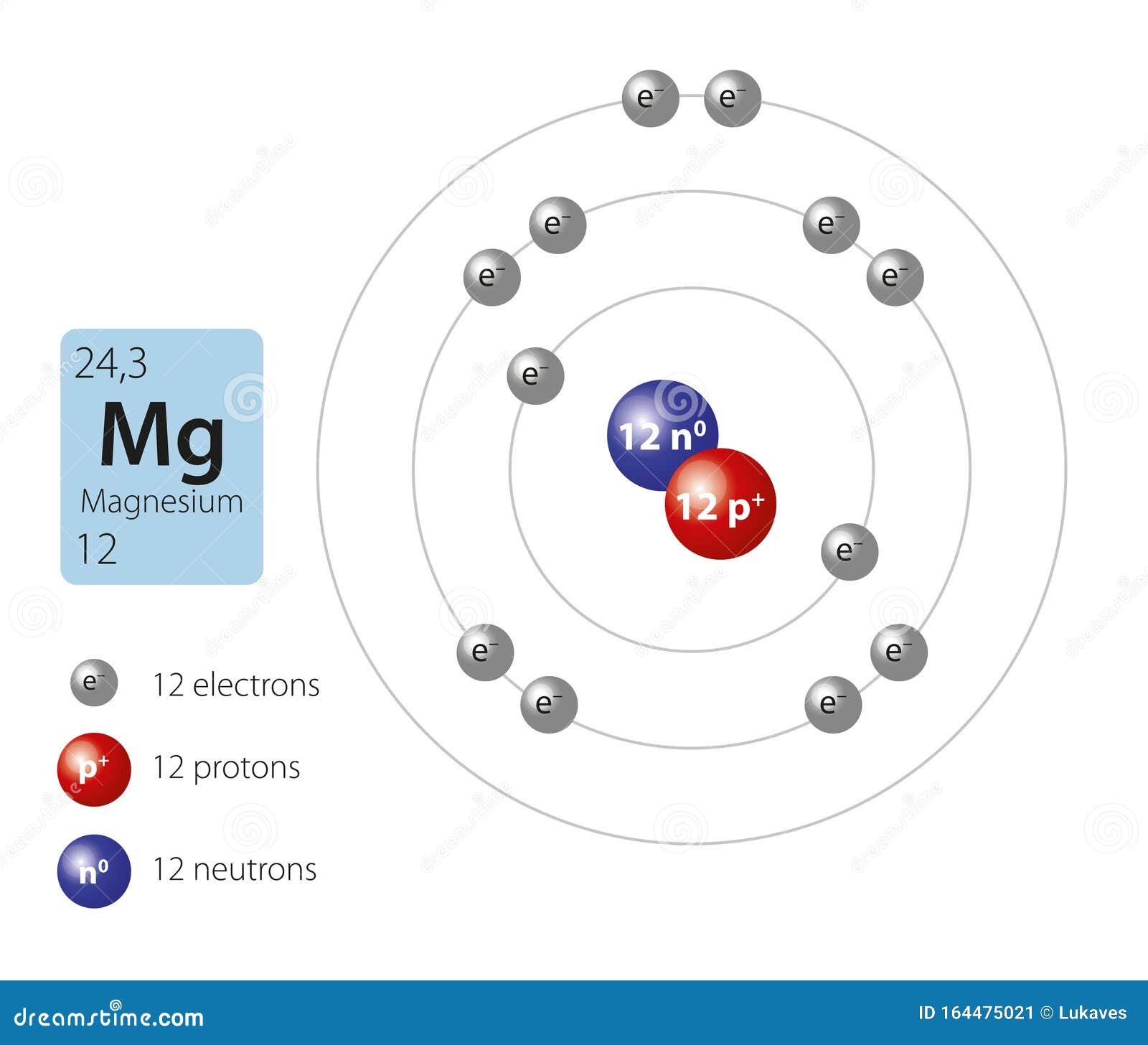
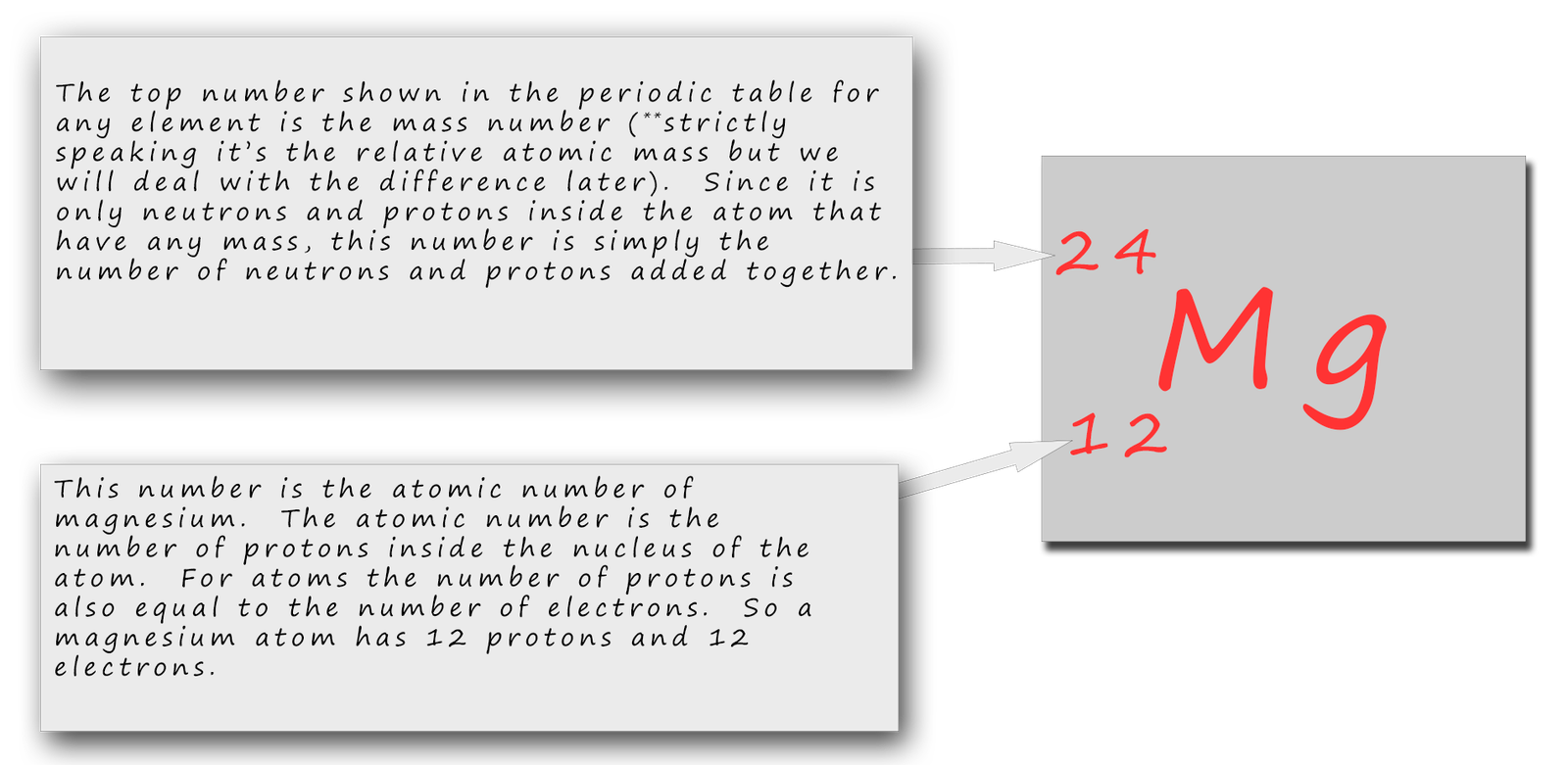
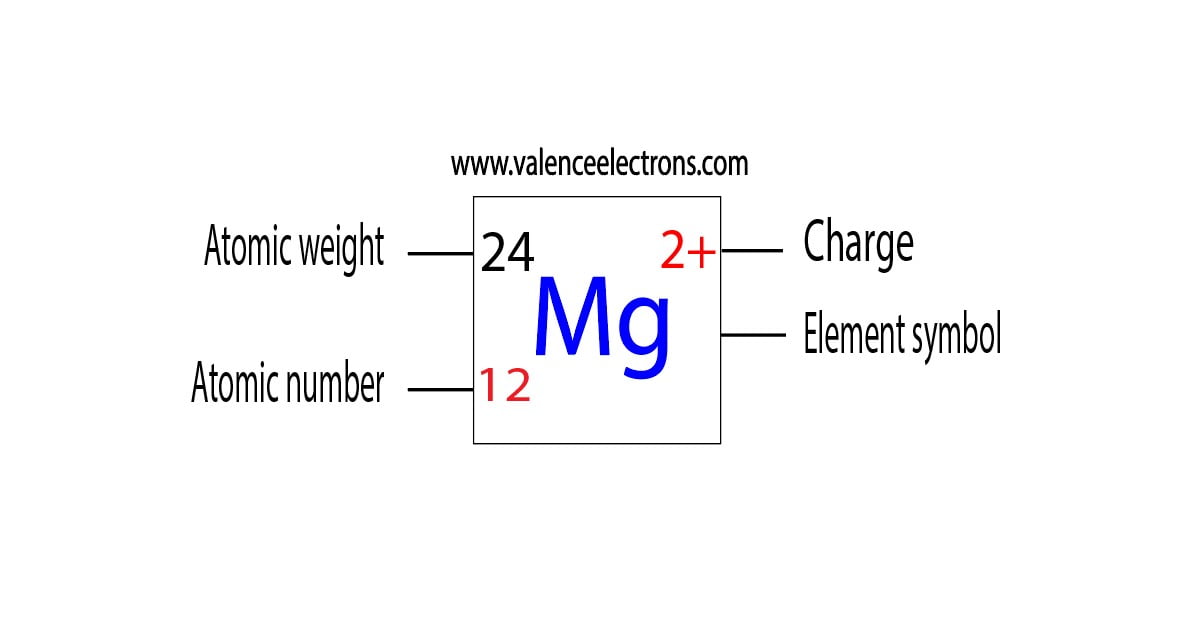
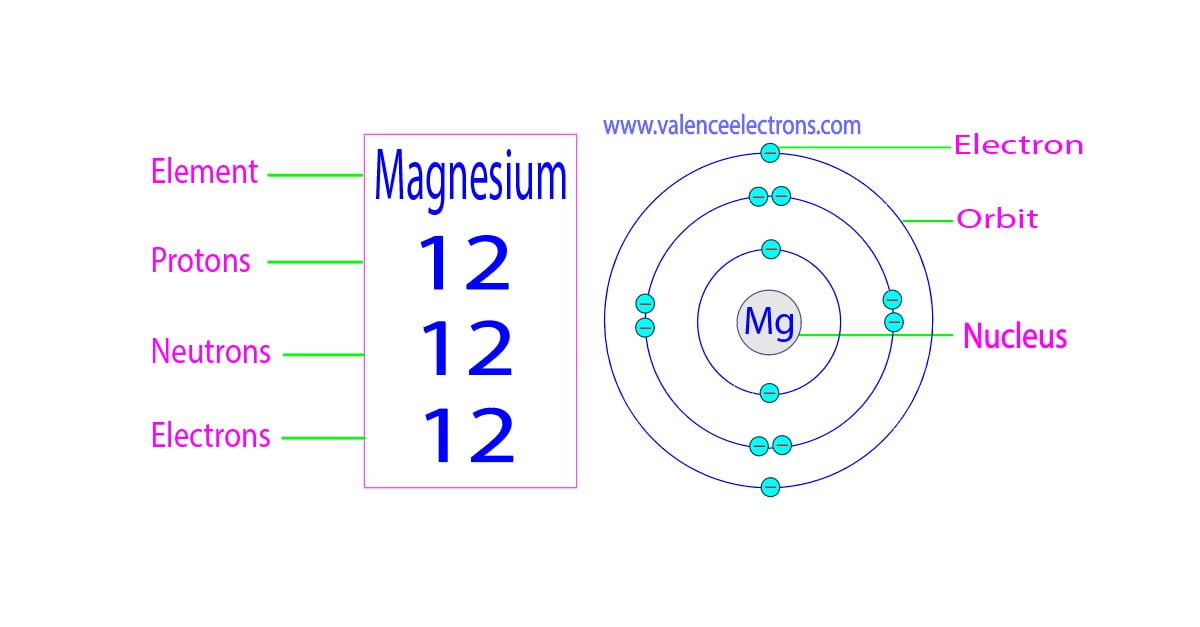
Magnesium is the 12th element of the periodic table so its atomic number is 12. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a magnesium atom has twelve protons and twelve electrons.
Magnesium Periodic Table and Atomic Properties
Protons and neutrons have masses of approximately 1 unified atomic mass unit (u) and cannot easily be split into smaller particles. Magnesium consists of protons, neutrons, and electrons and has an atomic mass of 24.3 u. Why is the atomic mass of magnesium significantly greater than 24 u?
Modelo Atomico Del Magnesio Compuesto
An isotope is a compound with the same number of protons and electrons, but different number of neutrons. The three most natural isotopes of Mg are Mg-24, Mg-25, and Mg-26. Mg-24 (12 neurtrons) is 78.9%, Mg-25 (13 neutrons) is 10% and Mg-26 (14 neutrons) is 11.01%, of all the Magnesium found in nature.
Protons, neutrons and electrons.
The chemical properties of Magnesium depend (nearly) entirely on the electrons, and therefore depend on how many protons there are, and not how many neutrons; add extra neutrons and it's still "Magnesium", but it may weigh more. A handbook of chemistry and physics lists all isotopes with between 8 and 16 neutrons.
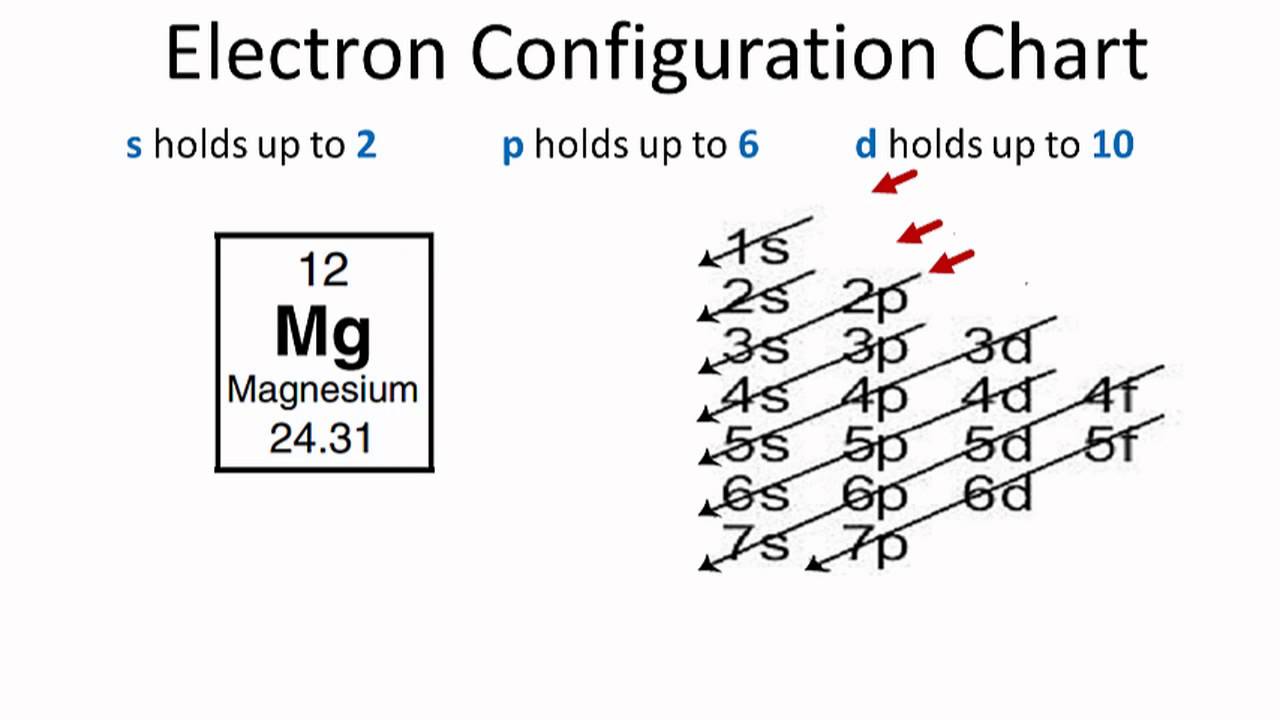
Magnesium(Mg) electron configuration and orbital diagram (2022)
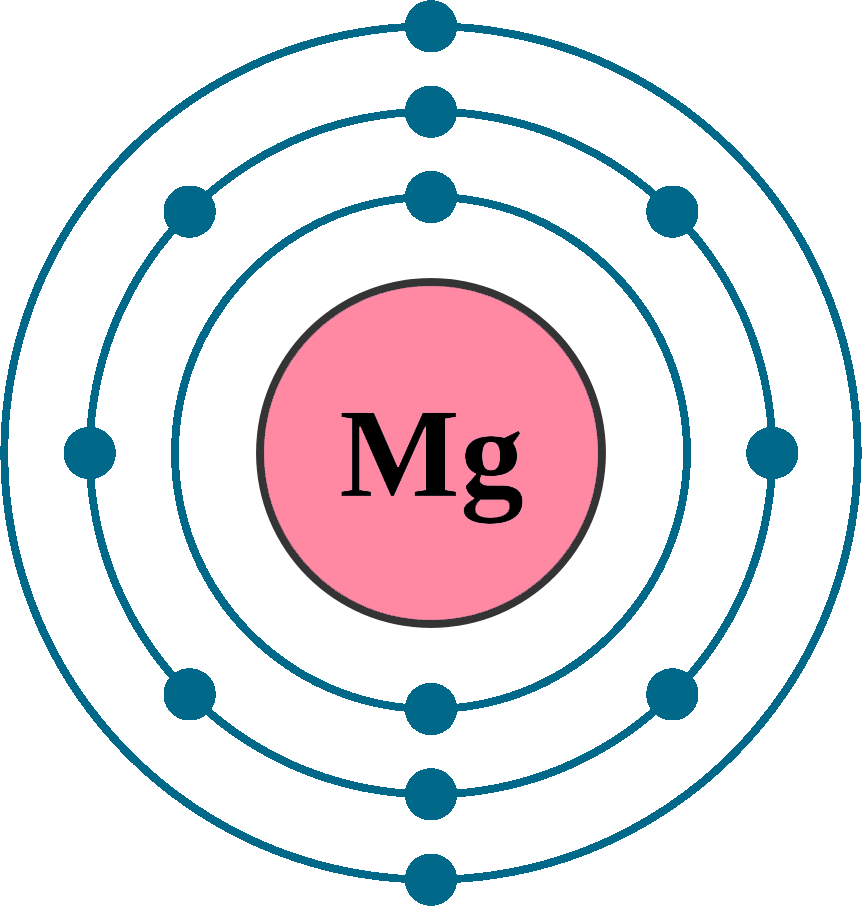
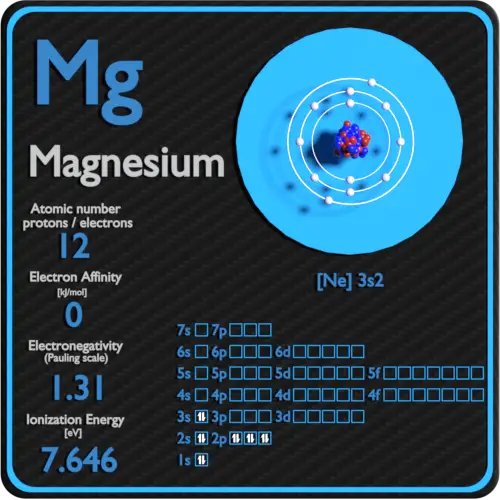
That is, magnesium is a cation element. Mg - 2e - → Mg 2+. The electron configuration of magnesium ion (Mg +2) is 1s 2 2s 2 2p 6. This electron configuration shows that magnesium ion (Mg +2) has acquired the electron configuration of neon and it achieves an octave full stable electron configuration.
subatomic particles Montessori Muddle
Summary Atomic Number - Protons, Electrons and Neutrons in Magnesium Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
magnesium electron configuration Newton Desk
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise. An ion of platinum has a mass number of 195 and contains 74 electrons.
3d render of atom structure of magnesium isolated over white background
Neutrons = atomic mass - atomic number. Magnesium neutrons. The atomic mass of magnesium is 24.305, so we'll take the roundup value as 24. And the atomic number of magnesium is 12. Subtract the atomic number (12) from the atomic mass (24). Hence, magnesium has a total of 24 - 12 = 12 neutrons.

How To Find The Number Of Neutrons In An Element redesigngreece
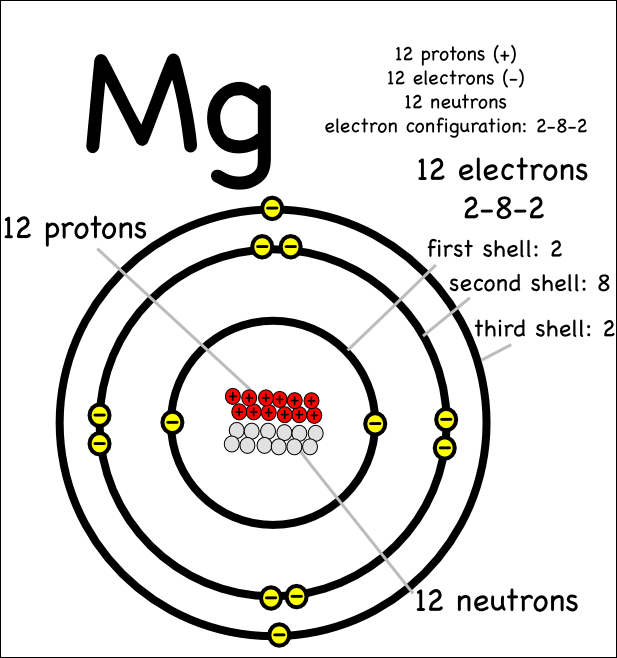
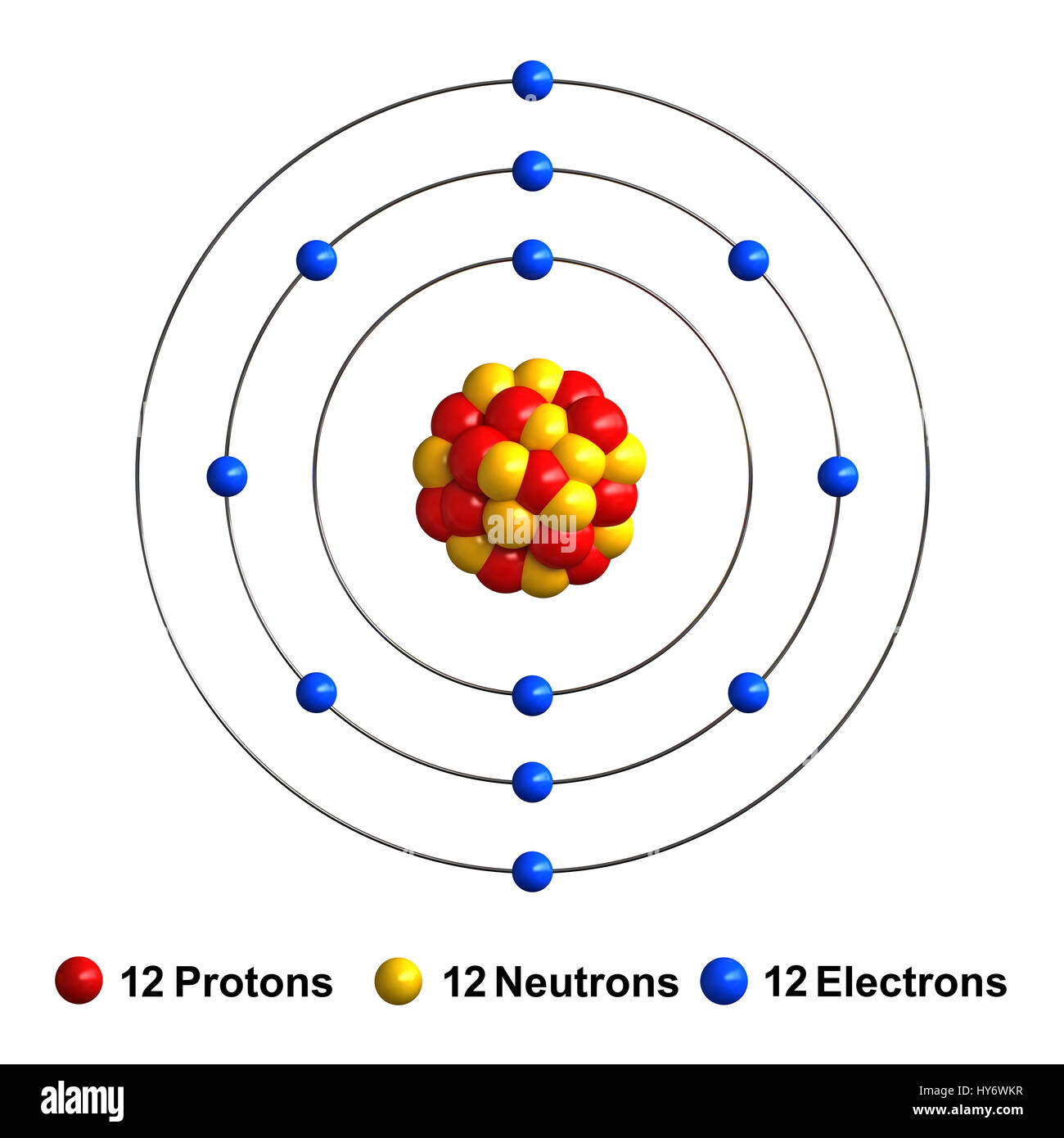
The most common and stable type of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons (which have a negative charge). An image of the secondary beam "cocktail" produced at a cyclotron center in Japan for a study of Mg-40, an exotic isotope of magnesium.

How many protons does an atom of magnesium have Geigade
Magnesium has 12 protons, 12 neutrons and 12 electrons. But how will you find the number of protons, neutrons and electrons in Magnesium (Mg)? Well, it is very easy to find the protons, neutrons and electrons of magnesium atom. Here I have given a very simple method for finding the protons, neutrons and electrons of magnesium atom.

Magnesium, atomic structure Stock Image C018/3693 Science Photo Library
Table 2.2 The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element: Its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

Magnesium Atomic Number Atomic Mass Density of Magnesium
Learn how to calculate the molar mass of Mg(OH)2, a common compound used in antacids and laxatives, by using the periodic table and a simple formula. This video tutorial will show you the steps.
Protons, Neutrons, Electrons for Magnesium (Mg, Mg2+)
There are 12 protons and 10 electrons in a "Mg"^(2+) ion, the normal amount of neutrons is 12. Magnesium is an element with atomic number 12. This means that every magnesium atom will have 12 protons. In a magnesium atom, there are 12 electrons, to make the atom have a neutral charge. When an ion is formed, the magnesium atom loses electrons. The 2+ charge shows that the magnesium ion has two.
Magnesium Periodic Table and Atomic Properties
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise 2.3.1. An ion of platinum has a mass number of 195 and contains 74 electrons.
Magnesium Electron Configuration (Mg) with Orbital Diagram
Element Magnesium (Mg), Group 2, Atomic Number 12, s-block, Mass 24.305. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.. The arrangements of electrons above the last (closed shell) noble gas.. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one.